The COV-GRIP® rapid antigen test is a CE-marked test for the rapid, qualitative and differential detection of the nucleocapsid antigen of influenza A (including the H1N1 subtype), influenza B and/or SARS-CoV-2.
A single sample gives 2 results.
In 15 minutes, COV-GRIP® highlights the absence or presence of antigens in the sample taken, as soon as the first symptoms appear. It has been validated by an expert laboratory.
A study carried out at the Virology Department of Hôpital Henri Mondor (AP-HP) evaluated 22 antigenic tests on more than 1,157 samples and 4 variants.
COVID-VIRO is considered to be :
“The reference test which showed excellent sensitivity and specificity (COVID – VIRO, AAZ) was used as a comparator “*.
In addition :
“The sensitivities of the 22 antigenic tests ranged from 59.7% … to 100% for AAZ” **
*Fourati, S. et al. Performance of 22 Rapid Lateral Flow Tests for SARS-CoV-2 Antigen Detection and Infl uence of Variants of Concern : Implications for Clinical Use. Microbiology Spectrum, 10(4). Août 2022.
** Ct ≤ 25
Packaging : box of 20 test

Legal information
COV-GRIP® is a rapid test for the rapid, qualitative and differential detection of influenza A core protein antigen (including H1N1 subtype), influenza B and/or SARS-CoV-2 for use by healthcare professionals.
This test is reliable for detecting infection with SARS-CoV-2 during the incubation period of the virus, which can range from 1 to 14 days but is generally 3 to 7 days.
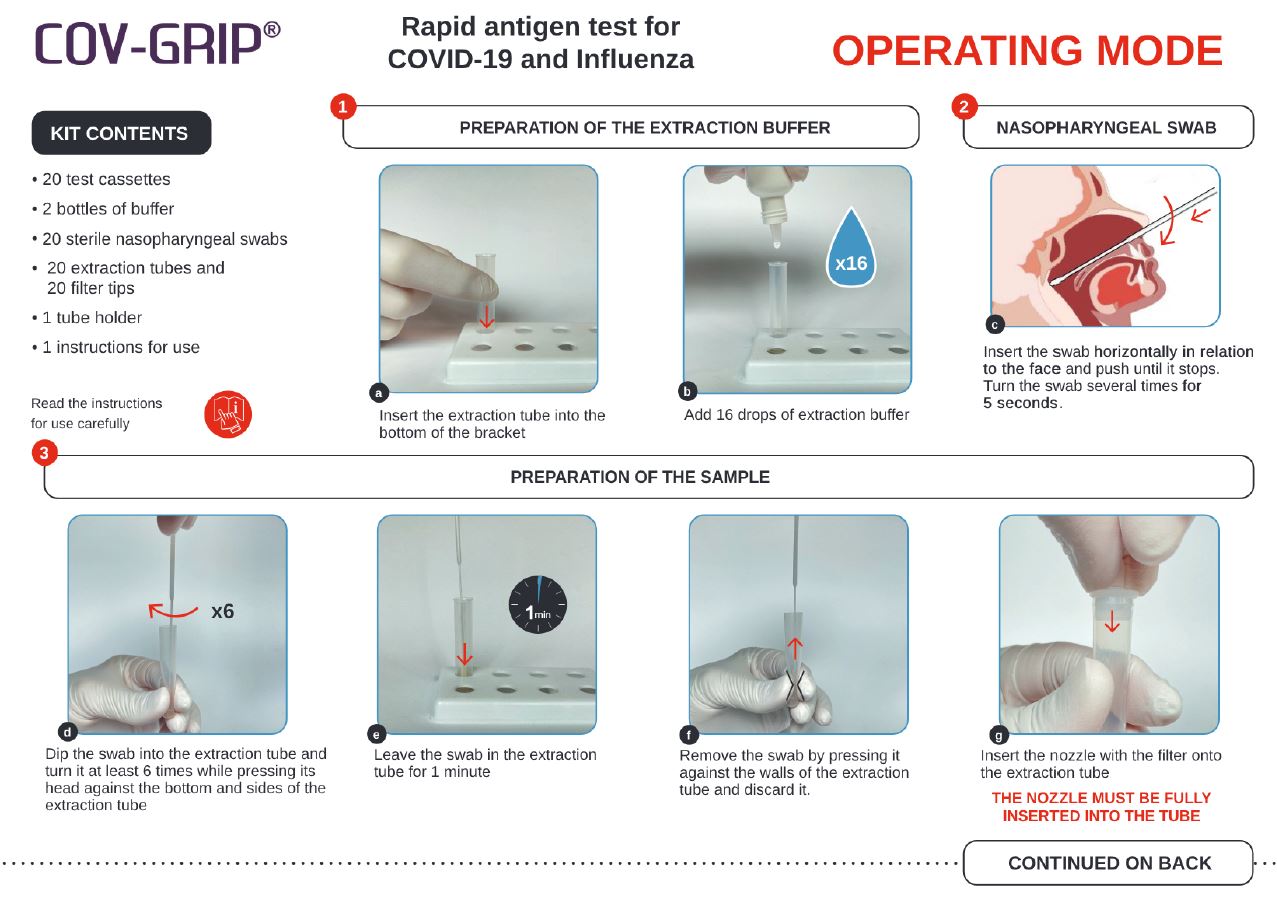
Read the instructions for use carefully.
Manufactured and distributed by : AAZ-LMB 43 rue de Bellevue 92100 Boulogne-Billancourt FRANCE
This in vitro diagnostic medical device is a regulated health product which bears the CE mark.
Page reference : AAZ.MKT.CG.02-A
Date : 7 November 2024